
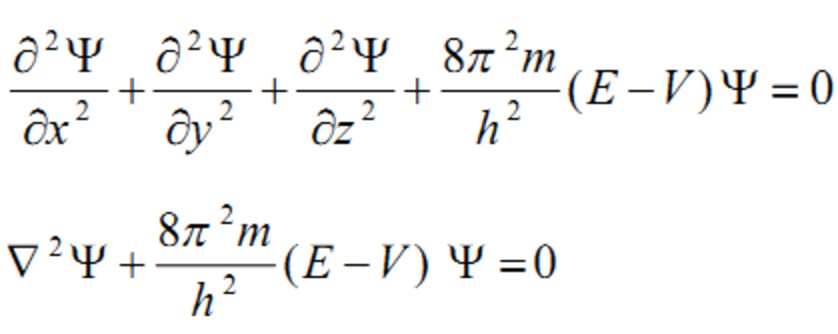
With its position and momentum completely unpredictable, even getting a boundary is a lot.

An electron that was considered a particle till then showed the properties of a wave.Īnother Physicist Max Born suggested that this wave was not a normal wave rather it was a probability wave wherein the probability of an electron existing at one place is dependant on the wave size.Įrwin Schrödinger came up with an equation that described these electron waves with pretty good accuracy. It resulted in electrons showing wave nature. The roots of this equation lie in the double-slit experiment on electrons performed by two physicists named Davisson and Germer. Schrodinger equation is a partial differential equation that describes the form of the probability wave that governs the motion of small particles, and it specifies how these waves are altered by external influences.


 0 kommentar(er)
0 kommentar(er)
