
Experiments have been made at different temperatures to obtain this data. The water viscosity to temperature chart above is a visual representation of the data recorded below. Below is a water viscosity to temperature chart that shows the effect of temperature on the dynamic viscosity and kinematic viscosity of water. This value is the viscosity of water at 20☌. The dynamic viscosity of water at room temperature has a value of around 1.0 mPa⋅s, and it decreases as temperature increases. Water, being the most studied liquid, is the best fluid to start with when learning about viscosity. You can use our pressure converter and area converter for these procedures, especially if you have a lot of values to convert. However, if you need to express the viscosity of water in English units, you can always convert the milliPascal part to pound-force per square foot, and the square millimeters to square inches, for the dynamic viscosity and kinematic viscosity respectively. We can measure dynamic viscosity in millipascals-second (mPa⋅s) or with a fancier equivalent called the "centipoise." On the other hand, we can express kinematic viscosity in square millimeters per second (mm 2/s), which also has an equivalent unit called "centistokes." For the simplicity of this text, we will only be using milliPascals-second and square millimeters per second for dynamic viscosity and kinematic viscosity, respectively. On the other hand, the kinematic viscosity tells about the speed the fluid reaches when a particular force is applied to the fluid. When choosing between the two viscosities, it is worth noting that dynamic viscosity tells us about the force required to move the fluid at a certain speed. The larger the force or stress needed to move the plate, the more viscous the fluid is. Dynamic viscosity, or the absolute viscosity of water, or any fluid, is proportional to the tangential shear stress per unit area needed to move one plate at a constant speed over another plate at a maintained fluid thickness between these two plates, like in a Couette flow, as shown below: When dealing with viscosities, when we mention "viscosity," we actually mean dynamic viscosity. In this article, we'll focus more on the viscosity of liquids, specifically on the kinematic viscosity and the dynamic viscosity of water. This weakening results in liquid molecules to move more freely and, therefore, with a lower viscosity. In liquids, when molecules start to move faster, their attraction from each other weakens. As a result, in gases, molecules experience more friction against each other, making them flow slower and become viscous. However, when we apply heat or additional thermal energy to our fluids, their molecules start moving faster.
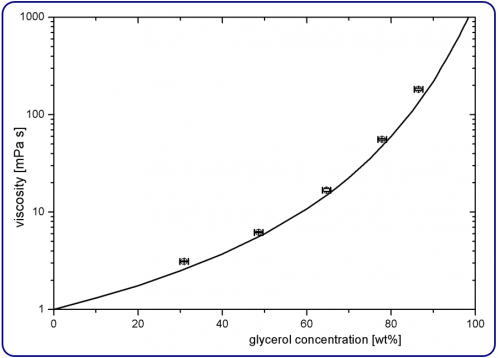
The attraction between the molecules of a viscous fluid is much higher than that of a less viscous fluid. We can also express viscosity as the internal friction of a fluid in motion. Maple syrup, a very viscous fluid, would pour slower than when you pour milk on your cereal as milk's viscosity is much lower.
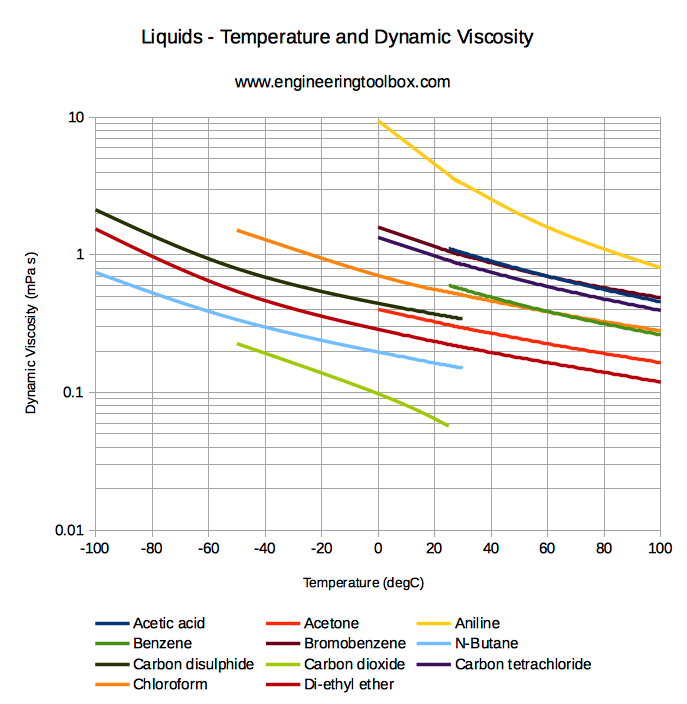
Imagine dripping maple syrup on your waffles for your breakfast.
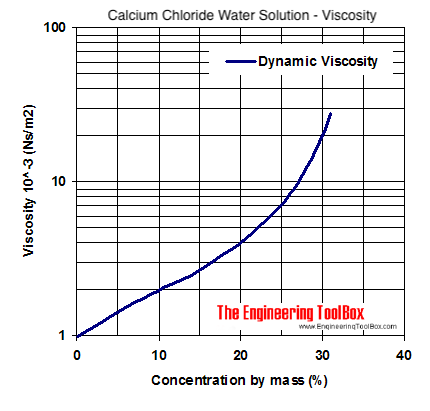
The higher the viscosity of a fluid (liquid or gas), the slower it traverses across a surface.

Viscosity is the measure of a fluid's resistance to flow.


 0 kommentar(er)
0 kommentar(er)
